Every year, tens of thousands of Americans lose their lives in what experts have described as the “worst crisis the United States has ever faced.”
But it could become a thing of the past thanks to a new vaccine that would be given to high-risk drug users months or perhaps years before an overdose.
The vaccine – which will likely be given via several injections – essentially teaches the immune system how to stop opioids from blocking oxygen to the brain.
The researchers say they expect human trials to begin “in early 2024.” The fentanyl vaccine would be different from the antidote Narcan, which is given during an overdose and has already been approved by the Food and Drug Association (FDA).

Molecular biologist Jay Evans of the University of Montana (above) expects his group’s vaccines targeting heroin and fentanyl to enter human trials “in early 2024”
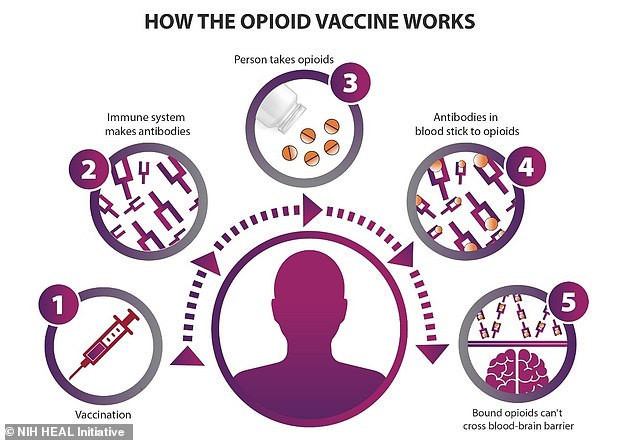
Both heroin and fentanyl bind to opioid receptors in areas of the brain that control pain and emotion. During an overdose, the brain becomes starved of oxygen, which kills neurons. An opioid vaccine would neutralize the chemical with antibodies while it’s still in the bloodstream
In animal studies, including one published last July in the journal Nature npj Vaccines, the researchers were able to show that such fentanyl vaccines could be boosted with the addition of a second small molecule called an adjuvant.
But these tests will need to be safe and repeatable in humans before anyone can get in line for their own injections.
The team, led by researchers at the University of Montana, actually created two new vaccines, with the second targeting heroin overdoses.
Both heroin and fentanyl attack and dull the areas of the brain that control pain and emotion. But during an overdose, these chemicals deprive the brain of oxygen, killing neurons.
The new vaccines, similar to concurrent fentanyl vaccine research elsewhere, are designed to neutralize opioid chemicals with antibodies while these compounds are still in the bloodstream.
“Once safety and early efficacy are established in these early clinical trials,” said molecular biologist Jay Evans of the University of Montana (UM), “we hope to develop a combined multivalent vaccine targeting both heroin and fentanyl.” “.
America is currently in the midst of a fentanyl epidemic, with about 150 Americans dying every day from the synthetic opioid, according to the US Centers for Disease Control and Prevention (CDC).
The CDC estimates that of the 107,081 overdose deaths reported in the United States in 2022, more than two-thirds or more than 72,000 could be attributed to synthetic opioids such as fentanyl.
Evans, who also co-founded the start-up Inimmune designed to bring these drugs to market, has focused his UM team on creating more adjuvants, substances they hope will further boost the effectiveness of vaccines.
“Our adjuvants enhance vaccine response, providing stronger and longer-lasting immunity,” said Evans, whose official title is Director of the UM Center for Translational Medicine.
Evans’ UM team patented the INI-4001 adjuvant, which will be just one aspect of the final vaccine cocktail.

Fentanyl has invaded some American communities and was responsible for 71,000 overdose deaths in 2021. Pictured: A homeless man in Seattle, Washington smokes fentanyl
“We have worked closely with researchers from Inimmune, the University of Minnesota, the University of Washington, the Hennepin Healthcare Research Institute and Columbia University over the past several years to design and optimize opioid vaccines for the advancement towards human clinical trials,” he said.
The vaccine itself works by stimulating the T cells of the immune system to make antibodies that bind to fentanyl in the blood.
These immune proteins capture the drug as it enters the body and prevent it from spreading further and causing harm. It is then processed in the kidneys and excreted from the body.
‘Antibodies produced through active immunization with an opioid vaccine sequester the opioid in the blood and prevent it from crossing the blood-brain barrier,’ according to Dr. Marco Pravetoni, Evans’ research partner at the University of Washington where he directs the Center for Drug Development for Substance Use Disorders.
“The first vaccine will target heroin,” Evans said, “followed shortly by a fentanyl vaccine in Phase I clinical trials.”
These Phase 1 human trials, he noted, will be led by team collaborator Dr. Sandra Comer of Columbia University in New York.
Finding the right subjects, patients currently using fentanyl or heroin, could take six months or more.
And the process will have to proceed slowly to protect the health and safety of these human subjects.
Phase 1 trials, Evans said, will involve very gradual increases in vaccine dosage.
“We’ll start with the lowest dose, a dose that may not be effective,” Evans said.
‘Phase I clinical trials focus on safety. Once the first dose cohort is complete, a data safety monitoring committee reviews the data and approves testing at the next dose level if the vaccine is safe. The process takes time until safe and effective dosage levels are reached.’
Evan added that Dr. Comer and others will also follow study patients to assess “how long the antibodies to opioids will last.”
If such trials prove effective and safe, Phase 2 human trials will face the challenge of pinpointing details such as the number of doses needed for effective overdose protection and the appropriate time required between doses.
Next would come Phase 3: a large cohort study with many participants designed to help the FDA decide whether the life-saving benefits of the vaccine outweigh the possible risks from side effects.
But a similar vaccine, targeting prescription opioidoxycodone, is already paving the way for heroin and fentanyl vaccines.
Evans’ research partner, University of Washington’s Dr. Pravetoni brought the oxycodone vaccine into Phase I trials with Columbia’s Dr. Comer in 2021.
“Our vaccines are designed to neutralize the target opioid, sparing critical drugs such as methadone, buprenorphine, naltrexone and naloxone,” Evans noted, “which are used in the treatment of opioid addiction and overdose reversal.”
That needle is threaded by so-called “hapten” and “drug conjugate” vaccines developed by Dr. Pravetoni, which can stimulate the body’s immune system to produce antibodies only against target opioids.
While these findings have shown great success in animal tests and other lab-scale experiments, Evans cautioned that there are many regulatory steps that need to be taken before these vaccines can begin helping users currently at risk.
“It takes many years to get to an approved final product,” he said.
“Based on the efficacy data we see in our preclinical data and the established safety profile in animal models, we are very confident that these vaccines will be successful. But there’s still a lot of work to do.”
#Vaccine #FENTANYL #heroin #overdoses #enter #human #trials #Injection #highrisk #addicts #months #advance
Image Source : www.dailymail.co.uk